JEE Main 2022 Important Questions With Solutions (200 Plus)
JEE Main 2022 Important Questions With Solutions Download Free PDF Here
JEE Main 2022 Important Questions : Students can find here JEE Main 2022 Important Questions with Solutions for preparation for the JEE Main Exam, We know that engineering entrance exams like JEE Mains, JEE Advanced, & other entrance exam are primarily based on the class 11th and class 12th syllabus which is huge. So, for all these exams we have identified JEE Main questions with Solutions and explanations from all the three subjects, i.e., Maths, Physics and Chemistry.
Q1. Find out the number of enantiomer, meso isomer and total number of optical isomers for the following compound
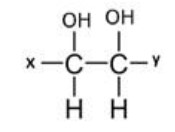
Options:
(a) 3,2,4
(b) 0,2,3
(c) 4,0,4
(d) 0,2,2
Ans. Option (c) is correct
Explanation:
Before solving the or guessing the options, you should check either the given molecule is
symmetrical or unsymmetrical
The given molecule is unsymmetrical as it has group X at one end and group Y at other end
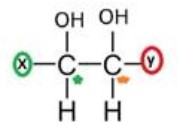
Then after you need to find out the number of chiral carbon
So, the number of chiral carbon in the given compound is: 2
Now come to formula to calculate the number of enantiomer, meso isomer and total number of
optical isomers
1. Number of enantiomer = 2n (n is the number of chiral carbon )
2. Number of meso isomer = ZERO
Q 2. The number of enantiomer in a glucose molecule is
Options:
(a) 16
(b) 12
(c) 4
(d) 9
Ans. Option (a) is correct
Explanation:
First you should look at the glucose structure
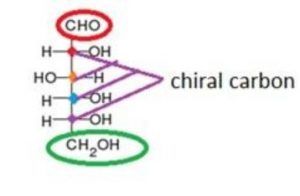
And the find out the number of chiral carbon which will be denoted by n
Here in Glucose molecule the number of chiral carbon is = 4, as shown in the figure
As we have seen the formula above to calculate the number of enantiomer, = 2
n
So, total number of enantiomer in Glucose molecule is: 2
4
= 16
Note:
• The combination of d(+) and l(-) isomer is known as enantiomer
• Enantiomer reacts with different speed with different chiral reagent
• The stereoisomers which are non superimposable mirror images of each other is known as
enantiomer
Q 3. Enantiomer can be separated by use of
Options:
(a) Enzymes
(b) Physical method
(c) Chemisorption
(d) Rotation
Ans. Option (a) is correct
Explanation:
Enantiomer has different biological properties and hence can be separated by enzymes
Q4. Select the correct stability order of the conformation /Rotamers/ Rotational isomers of Ethane
Molecule
Options:
(a) Partially Eclipsed > Fully Eclipsed > Anti-staggered > Skew or Gouche
(b) Skew or Gouche > Partially Eclipsed > Fully Eclipsed > Anti-staggered
(c) Anti-staggered > Skew or Gouche > Partially Eclipsed > Fully Eclipsed
(d) Fully Eclipsed > Anti-staggered > Skew or Gouche > Partially Eclipsed >
Ans. Option (c) is correct
Explanation:
• The isomers which can be produced by the free rotation about single bond are known as
conformation /Rotamers/ Rotational isomers and this phenomenon is called as conformational
analysis
• These isomers can be represented by
1. sawhorse projection
2. Newman’s projection formula
3. A staggered conformer is more stable than an eclipsed conformer as the latter involves
unfavourable energy interactions between atoms.
4. In conformational analysis (energetics study of different rotational conformers or rotamers),
structures are represented by *Newman projection * (atoms and bonds are viewed along the axis of
rotation).
Q 5. Beckmann rearrangement is not catalysed by
Options:
(a) H2SO4
(b) P2O5
(c) BF3
(d) NaOH
Ans. Option (d) is correct
Explanation:
Beckmann rearrangement is only catalysed by Acid catalyst and used to determined the
configuration of ketoximes
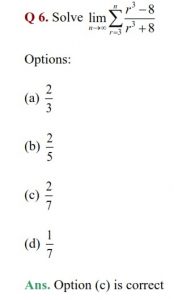
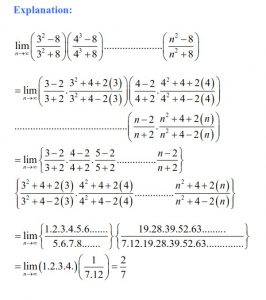
Q 7. Which of the following statements is not correct about order of a reaction?
Options:
(a) The order of a reaction can be a fractional number
(b) Order of a reaction is experimentally determined quantity
(c) The order of a reaction is always equal to the sum of the stoichiometric coefficients of reactants
in the balanced chemical equation for a reaction
(d) The order of a reaction is the sum of the powers of molar concentration of the reactants in the
rate law expression
Ans. Option (c) is not correct
Explanation:
Order of reaction is equal to the sum of powers of concentration of the reactants in rate law
expression.
For any chemical reaction
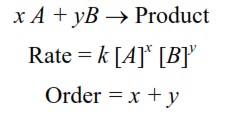
Order of reaction can be a fraction also. Order of reaction is not always equal to sum of the
stoichiometric coefficients of reactants in the balanced chemical equation. For a reaction it may or
may not be equal to sum of stoichiometric coefficient of reactants.
Q 8. In a reaction if the concentration of reactant A is doubled, the rate of reaction becomes eight times. What is the order of the reaction?
Options:
(a) 2
(b) 3
(c) 8
(d) 4
Ans. Option (a) is correct
Download 200 Plus full JEE Main 2022 Question With Solutions PDF